If you’re curious about or are considering in vitro fertilization (IVF) as a way to add to your family, you might be wondering what exactly the process involves. The truth is that IVF is a multi-stage process, and the exact steps of what is required (or what you choose) can vary between individuals.
Treatment can differ depending on whether you are diagnosed with a certain condition like polycystic ovary syndrome (PCOS) or if the eggs involved are from a donor or a woman of advanced maternal age.
In general, the process of IVF involves:
- Hormone treatment to grow as many follicles as are available
- Retrieving eggs
- Fertilizing them in a laboratory
- Transferring the resulting embryo(s) back to a uterus or freezing them for future use.
One cycle (from hormone injections to embryo transfer) can take between 4 to 6 weeks. Here we’ll take an in-depth look at IVF and explain the process and options that may be available to you in greater detail.
Stimulation Protocols
Each menstrual cycle, women will have multiple follicles, which are small fluid-filled sacs containing an immature egg. In a natural menstrual cycle, one follicle will be selected to become the dominant follicle. It will respond to follicle-stimulating hormone (FSH) and other hormones released from the brain, causing the follicle to increase in size.
The egg inside the follicle will develop and mature. Eventually, the luteinizing hormone (LH) surge will occur and cause the egg to ovulate from the ovary.
During IVF, fertility drugs are used to stimulate a woman’s ovaries to produce multiple follicles (each of which contains a single egg) intending to retrieve multiple eggs during the egg retrieval.
A higher number of follicles during IVF stimulation usually translates to a higher number of eggs being retrieved and hopefully a better chance of having high-quality blastocysts available for embryo transfer.
Variation in Stimulation Protocols
There are different ways medications are used to stimulate the ovaries during an IVF cycle. These are called IVF protocols. If a woman is a poor responder, meaning she has a lower ovarian reserve than is expected for a woman her age, she may be on a different stimulation protocol than a woman with polycystic ovaries who has a lot of egg follicles. (You can read more about this in IVF Stimulation Protocols.)
There are a few possible stimulation protocols. It may take more than one cycle of fertility drugs to develop the proper response in terms of the number and size of follicles that develop. It might even be necessary to cancel a cycle after starting the fertility medications and restart on a different protocol.
Although this may be a disappointing result, remember that you are starting over with a better understanding of your body.
Related:
Triggering the Release of Mature Eggs
Fertility drugs will stimulate the growth of the follicles, and their growth will be monitored by frequent ultrasounds and blood tests to check your hormones during the stimulation process.
Once the follicles in the ovary reach the appropriate size (generally around 10 days after fertility drugs were started), an injection of synthetic human chorionic gonadotropin (hCG) hormone called a trigger shot is given to mature the eggs and release them from the wall of the follicle. (The eggs will stay inside the follicle and not be ovulated where they remain until egg retrieval.) Maturation of eggs may also be done with GnRH agonists known as Lupron.
The timing of this injection is critical as the eggs should be allowed to mature as much as possible before they are removed through the egg retrieval procedure. The shot is usually administered 34-36 hours before an egg retrieval procedure.
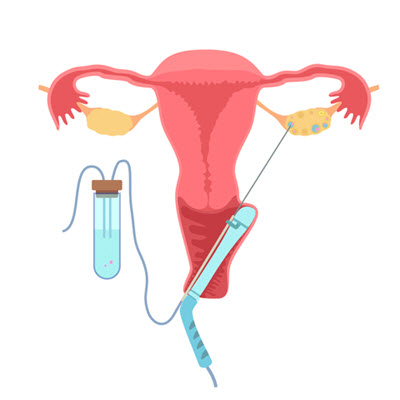
The Egg Retrieval Procedure
Once the follicles have reached a specific size and the eggs have matured, they need to be removed from the body. This process of removal is called an egg retrieval procedure and is performed by your IVF doctor and a team of nurses. (Read more about preparing for your egg retrieval.)
Most egg retrievals are done under light anesthesia and take approximately 10-15 minutes to complete. Most women do well after the retrieval and report mild to moderate cramping, similar to menstrual cramps.
After the procedure is complete, it is advisable to rest and not plan on returning to work or other activities.
An egg retrieval process generally includes the following steps:
- The doctor inserts a long hollow needle into the ovary through the wall of the vagina with the help of ultrasound guidance.
- When the needle punctures a follicle, suction is applied, and the contents of the follicle, including the microscopic egg, are drained and transferred to the embryologist.
- The embryologist identifies and isolates the eggs from the follicular fluid.
Expectations After Your Egg Retrieval
After the egg retrieval, it is only natural to have questions about the degree of success of the procedure and what that means for your odds of achieving pregnancy. Here are a few tips to keep in mind to help adjust your expectations for the amount of post-retrieval info you can expect.
- The number of follicles seen on ultrasound is only the approximate number of eggs you can expect to retrieve; sometimes there are more, and sometimes there are fewer.
- Not all of the eggs retrieved will be mature, and the immature eggs cannot be fertilized and will not be usable. Additionally, not all mature eggs will fertilize, and not all fertilized embryos will develop normally. Thus there is a natural attrition rate with IVF.
- On the day of the egg retrieval, little information about the quality of the eggs is available. At most, we can tell the number of eggs and possibly how many are mature. Most eggs look identical at this stage. The difference in the quality of eggs becomes evident only in the days after fertilization when any fertilized eggs have started to divide and grow into embryos.
Semen Samples for an IVF Procedure
Once the eggs have been extracted, they need to be fertilized. This requires fresh or frozen sperm from a partner or donor. If the male partner is giving a fresh semen sample on the day of the egg retrieval process, he will likely be asked to do so around the time of the egg retrieval procedure. Alternatively, a sample may have been previously frozen.
The lab prepares the sperm by separating the sperm from the seminal fluid in a process known as sperm washing.
Abstinence Before Producing a Semen Sample
It is generally recommended that the male partner abstains from ejaculating for two or three days before the egg retrieval. Most clinics will have a recommendation on optimal abstinence time based on the evaluation of a semen sample.
Studies have shown that frequent ejaculation can improve sperm quality (fewer dead and non-motile sperm) and reduce DNA fragmentation, which can contribute to poor-quality embryos.
Egg Fertilization: Insemination or ICSI
Two possible methods of fertilization may occur as part of an IVF procedure. Either natural insemination or intracytoplasmic sperm injection (ICSI) is performed to fertilize the eggs, generally on the day of the egg retrieval.
- Natural Insemination
Insemination involves placing several thousand sperm around the eggs and allowing them to fertilize naturally. They are left overnight and examined the next morning for signs of fertilization.
- Intracytoplasmic Sperm Injection (ICSI)
If there is any concern about the sperm’s ability to fertilize the eggs, the embryologist may decide to perform intracytoplasmic sperm injection (ICSI). This procedure involves selecting a single, normal-appearing sperm with a high-power microscope and injecting it directly into an egg. This is considered to be an effective method with fertilization rates of 60% to 80%.
This procedure is used in cases where the male partner has:
- Low sperm count
- Low sperm motility (movement)
- Low sperm morphology (shape)
- Surgically extracted sperm
- Vasectomy reversal
Or in cases of:
- Previously low fertilization rate
- Unexplained infertility
Some clinics exclusively perform ICSI on all their patients to avoid the rare case of total fertilization failure and, therefore no embryos for transfer.
According to the American Society for Reproductive Medicine (ASRM), in cases where male factor infertility isn’t an issue, there is no data to support the routine use of ICSI.
Stages of Embryonic Development
Once fertilization has taken place, the wait is on to determine the quantity and quality of developing embryos. Here we provide an overview of what you might expect after the egg retrieval has taken place. (Read in more detail: 6 Days in the IVF Lab)
Day 1: The Fertilization of Retrieved Eggs
The day of fertilization is counted as Day 1 of embryo development. On this day, your embryologist will check to see if any of the eggs were successfully fertilized overnight. On average, approximately 80% of the mature eggs fertilize normally. On this day, you should find out how many embryos you have. The lab staff or one of the nurses will likely call you with this information.
Fertilization is evident by the appearance of two circles inside the egg called pronuclei. One circle contains the genetic material from the sperm, and one contains the genetic material from the egg. These pronuclei fuse within a few hours. At this stage of development, the embryo is referred to as a zygote and is one single cell.
Sometimes more than one sperm enters the egg, and there are three or more pronuclei in a single egg. These are carefully isolated from the other fertilized eggs because they cannot implant and grow normally.
Most patients are curious about the quality of the embryos. At this stage, the embryos look similar, and it’s not yet possible to distinguish embryo quality. The embryologist will likely not have much additional information to share besides the number of fertilized eggs.
Days 2 and 3: Cleavage Stage
On Day 2 of development, we expect the embryos to be subdivided into two or four smaller cells. This process of division is called cleavage.
On Day 3, embryos should be further divided into six or eight cells.
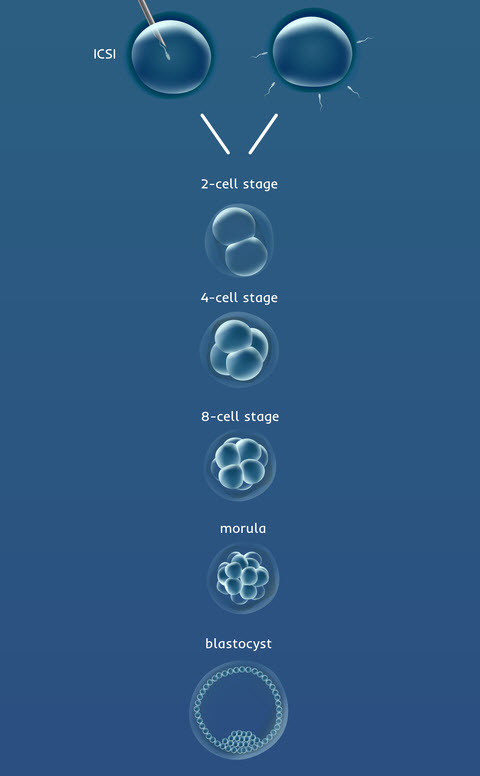
Day 4: Transition and Morula Stage
Following the eight-cell stage, the cells of the embryo merge to form what is called a morula. Viewed through a microscope, the embryo looks almost like a single cell again because the cells have merged. To merge, the cells have to express the correct molecules on the surface of the cells. It is the good-quality embryos that have a greater ability to undergo this process of merging.
Why did my embryos stop growing?
Between Day 3 and Day 5 of development, many important changes happen. This is the stage when an embryo is most likely to stop growing or “arrest development.” One reason for this is that after Day 3, the male chromosomes begin to contribute to the embryo’s development. Before this stage, the cell divisions are powered by only the egg’s energy. Any abnormalities in the sperm may slow down or stop the embryos from growing past Day 3.
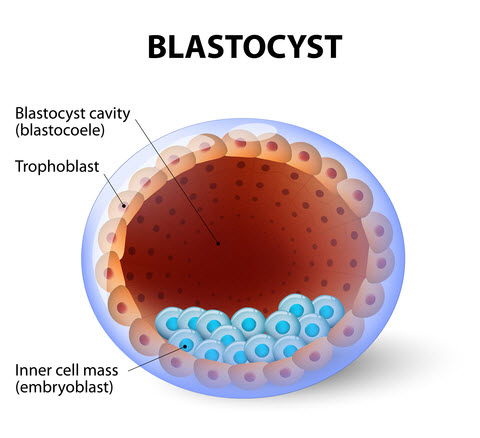
Days 5 and 6: Blastocyst Development
Following the morula stage is the all-important blastocyst stage, when the embryo takes in fluid to form a cavity, and the cells begin to differentiate into two different types. These two types of cells are called:
- Trophectoderm (T) – The T cells are a single layer of cells around the circumference of the embryo that gives rise to the placenta and embryonic sac.
- Inner cell mass (ICM) – The ICM is a distinct clump of cells that form the actual baby.
The overall structure of the blastocyst is important, as is the presence of these two different cell types. Because the appearance of the embryo at this stage is dramatically different from earlier stages of development, blastocysts have their separate grading system.
Blastocyst Hatching
The zona pellucida (protective shell around an egg) has two important functions:
- It allows only one sperm to enter the egg during fertilization, thereby maintaining the genetic integrity of the embryo (one sperm and one egg combine to make the perfect number of chromosomes).
- It holds together all the cells at the early cleavage stages (cell dividing) of development.
For a blastocyst to properly implant within the uterine lining, it must “hatch” from its shell. Usually, this happens on late day 5 or day 6 of development when the embryo takes fluid into the cavity and becomes too big for the shell to take the strain of the growing embryo. The blastocyst pulses, contracting and expanding until it gradually squeezes out of a hole in the zona pellucida.
The hatched blastocyst now has molecules on its surface that recognize and bind to molecules on the cell surface of the uterine cells to aid in implantation of the embryo into the uterus. Normal blastocysts implant around day 6 of development by coming into contact with the cells of the uterine lining.
Assisted Hatching
If the shells of the embryos are thicker than usual, it may be difficult for blastocysts to hatch naturally. In such a case, the embryonic cells cannot come into contact with and stick to the uterine lining. As a result, implantation fails.
Assisted hatching is a procedure where a hole is made mechanically or chemically in the shell of the embryo before embryo transfer. It can be done in several ways but is most often done using a laser mounted on a microscope.
Assisted hatching is often standard practice at most clinics and is used in all cases of frozen/thawed embryos and with patients who meet the following criteria:
- Have a raised follicle-stimulating hormone (FSH) level
- Are over 38 years of age
- Have embryos graded as poor-quality
- Have thickened shells around the embryos
- Have previously failed attempts at IVF
Several studies suggest assisted hatching may increase the chance of achieving pregnancy in patients with a poor prognosis or prior failed IVF cycles. Still, there is insufficient evidence that it increases live birth rates.
Assisted hatching is not a procedure that is well-suited for everyone. These same studies have shown no benefit from hatching embryos when comparing two similar groups (age, diagnosis) of patients in a study. The American Society of Reproductive Medicine does not recommend assisted hatching for all IVF patients.
Embryo & Blastocyst Grading
Embryos and blastocysts are graded to select the most promising for transfer. It is worth noting that each clinic has its grading system, so you will need to ask what system is used at your clinic to fully understand how grading may be applied to your embryos. Read more about Embryo and Blastocyst Grading.
Keep in mind that although low-grade embryos have a lower chance of implanting than their higher-graded counterparts, it’s still possible to achieve a pregnancy from embryos that are not ideal when viewed under a microscope.
Genetic Screening
An option for couples undergoing IVF is to have all the embryos genetically tested before embryo transfer. This can be done to reduce miscarriage rates by transferring only genetically normal embryos or to eradicate a genetic disorder from a family—for more detailed information read An Intro to Preimplantation Genetic Screening.
Embryo Transfer
Medication is taken to help prepare the uterine lining to be receptive to an embryo. The embryo transfer is performed by loading the embryos into a very fine catheter (or tube) and inserting it through the cervix into the uterus. The embryo is gently expelled from the catheter with a syringe and placed at the top of the uterine cavity. This is usually done with ultrasound guidance.
The embryo transfer should be painless; anesthesia is not necessary for the majority of women. However, you may be offered a muscle relaxant, such as Valium, to minimize uterine contractions following the procedure.
After the Embryo Transfer
After the embryo transfer, you should receive instructions before leaving the clinic. These instructions will include:
- What medication to take
- When your pregnancy test will be
- Any lifestyle restrictions such as exercise or abstaining from alcohol or caffeine
Clinics vary in the extent of restrictions following the transfer. As a general rule, take it easy, at least until you hear your results from the pregnancy test.
Your Pregnancy Test After IVF
You will most likely receive the results of a pregnancy test around two weeks after your embryo transfer. This period is often referred to as the two-week wait. (See our best tips for surviving the two-week wait after infertility treatment.) On the day of your pregnancy test, you will most likely have a blood (or potentially urine) test to detect the level of human chorionic gonadotropin (hCG).
A positive level indicates that the embryo has implanted. A follow-up hCG level will be performed two days later to see if the pregnancy is progressing normally; the hCG level should approximately double every 48 hours.
Unfortunately, not all embryos develop normally, and some pregnancies end in miscarriage. Your reproductive endocrinologist will follow the hCG blood levels early in the pregnancy, followed by one or two ultrasounds between six and 10 weeks of pregnancy.
Whatever the outcome of your fertility treatment, it’s essential to stay on all prescribed medication and follow your doctor’s instructions. A normal pregnancy will be followed until approximately 8 to 10 weeks gestation at the IVF clinic, at which time you will be referred to a regular obstetrician for prenatal care.
Early Outcomes From an IVF Cycle
IVF outcomes are individual. Personal circumstances include maternal age, the quality of embryos, and other physiological considerations. These have a considerable impact on the outcome of an IVF cycle. Early outcomes that may be expected from an IVF cycle include the following:
| Outcome |
Description |
| Pregnant |
Normal levels of hCG doubling appropriately followed by ultrasound at 6-7 weeks detecting a heartbeat and growing gestational sac. |
| Not pregnant |
A negative or undetectable level of hCG. You stop all medications and meet for a follow-up appointment to discuss the cycle and formulate a plan for the future. |
| Chemical pregnancy |
A chemical pregnancy or missed miscarriage is when an initial positive hCG level is followed by an abnormal rise or fall in hCG levels. Pregnancy loss happens before a gestational sac is detectable by ultrasound. |
| Blighted ovum |
A blighted ovum involves an initial positive hCG level followed by a normal or abnormal rise in hCG levels. Ultrasound reveals an empty gestational sac and no heartbeat. Follow-up ultrasounds confirm pregnancy is nonviable. |
| Ectopic Pregnancy |
An ectopic pregnancy is when the embryo grows outside of the uterus, usually in the fallopian tube but possibly also in the cervix, ovary, or bladder. This is a potentially dangerous situation and must be followed carefully by the doctor. The hCG level may start low and double slowly, but this is not always the case. Ectopic pregnancies can be detected via ultrasound and treated with medication or surgery. Ectopic pregnancies are nonviable pregnancies. |
Is IVF Right for You?
Hopefully, the information we have provided will help you to better understand what is involved in an IVF cycle. As detailed above, it’s an involved process that can be physically, emotionally, and financially taxing.
Get educated and consult with medical professionals to decide what’s right for you.
